This is a beta version of OpenPrescribing Hospitals for testing and feedback purposes. Data and functionality may be incomplete. Please contact us if you find any issues or have feedback. Sign up for email alerts to stay updated.
Frequently Asked Questions
Below are some answers to common questions about the OpenPrescribing Hospitals platform. If you can't find the answer to your question here, or would like to provide feedback on the platform, please contact us .
Contents
Platform
What does beta mean?
The platform is currently in beta, which means it's made available early to allow us to gather feedback and improve the platform. This also allows you to get an early look at the platform and helps us build a community of users to drive the development of the platform.
This does mean that you may encounter occasional issues or features that are still being refined. Please let us know if you encounter any issues or have any feedback.
How can I keep up to date with the platform?
You can sign up for email alerts to receive updates about the platform, including new data releases and feature announcements. See the Contact page for other ways to stay in touch.
Data source
Where do you get the data from?
The primary data source for the OpenPrescribing Hospitals platform is the Secondary Care Medicines Data (SCMD). The SCMD is collated by Rx-info and hosted on the National Health Service Business Services Authority (NHSBSA) Open Data Portal.
There is detailed user guide provided on the NHSBSA Open Data Portal alongside the data but briefly the SCMD contains processed pharmacy stock control data. Data is collected and curated from individual trusts by Rx-info. Rx-info then shares the data with the NHSBSA who make it openly available.
Other data sources which are linked to the SCMD to provide supplementary detail on the products and organisations within the SCMD, are described on the About page.
How often is it updated?
The SCMD is published monthly by the NHSBSA. Data is published with a 2 month delay, allowing for aggregation and curation of the dataset by Rx-Info prior to publication. The date of each release is available via the NHSBSA. This data is made available on the OpenPrescribing Hospitals platform shortly after publication by the NHSBSA. Sign up to our alerts email to be notified when new data is available on the site.
New data is initially made available as provisional data (see What is provisional data?). You can check the status of data available for analysis on OpenPrescribing Hospitals using our Submission History page.
What is provisional data?
Monthly data within the SCMD is initially published as provisional data. Provisional data is subject to change as Trusts submitting to the SCMD can update historical issues to reflect actual use. This is known as backtracking and affects 45% of the NHS Trusts submitting data to the SCMD. Backtracking is at its greatest in the most recent 3 months of data. Two months after the close of the financial year - once backtracking is accounted for - data is published as finalised data.
To see the data available or to read more about how it is made available please refer to the NHSBSA Open Data Portal where the SCMD is hosted. Alternatively we provide a summary of the latest data availability on our Submission History page.
Data contents
What is stock control data?
Stock control data is information collected from hospital pharmacy systems that is used to track and manage medicines use within hospitals. These systems support management of inventory levels, monitoring of medication usage and tracking of costs.
Unlike primary care prescribing data, hospital stock control data is not based on individual patient prescriptions. The data represents medication distribution within hospitals, such as issuing of medicines to wards or clinical areas, rather than actual patient-level prescribing.
Which medicines and devices are included?
All medicines and devices used by NHS Trusts in England with a dm+d code are included. You can view detailed information about all included products using the Product Lookup page.
What is the dm+d?
The dm+d is the standard dictionary for medicines and devices used across the NHS and contains standardised codes, descriptions and metadata for individual items. You can read more about how we use it on OpenPrescribing Hospitals in our blog post, Getting more from the secondary care medicines data using the dictionary of medicines and devices.
Is homecare medicines data included?
Some medicines that are issued by hospitals can be delivered directly to patients' homes. The official release guidance for the SCMD does not indicate whether this data is included in the dataset. However, in the methodology of the NICE Technology Appraisal Innovation Scorecard, which uses the SCMD and is published by the NHS Business Services Authority says the following:
Although the secondary care database captures some data for drugs supplied through homecare services, it is incomplete. Therefore, the actual volume of medicine used may be higher than the volume reported in the observed use.
If you know more details about homecare medicines in the SCMD, please let us know.
Why can't I find the medicine I am looking for?
There are several possible reasons:
- You're searching for a brand name which are not available in the SCMD. Search for generic names of the medicine you're looking for.
- It might not be issued in hospitals. Some medicines are only used in primary care.
- It might not have been prescribed within the NHS before.
- What you're looking for is a non-standardised item - where specific medicines do not have a dm+d code, they cannot be standardised across organisations and are not included within the SCMD. Examples include clean room consumables and packaging items.
- The medicine is dispensed by community pharmacy. NHS prescriptions supplied by hospitals but dispensed in community pharmacy are not included in the SCMD, but this data is available separately. This may be incorporated into the OpenPrescribing Hospitals platform in the future.
Is data for biosimilars available?
Products are reported in the SCMD at the level of Virtual Medicinal Products. This does not give any indication of whether a product is issued as a biosimilar or not. This would require products to be reported at the level of Actual Medicinal Products (AMPs), which are atributed to specific manufacturers. You can read more in our blog post, You asked: Can we monitor the usage of biosimilars in secondary care using OpenPrescribing Hospitals?.
Which NHS Trusts are included?
The platform includes data from all NHS hospital trusts in England that submit data to Rx-Info. This includes all NHS Acute, Teaching, Specialist, Mental Health and Community Trusts in England (this does not include medications dispensed by community pharmacy but this is available separately).
Why can't I find my hospital on the platform?
There are multiple reasons your hospital may not be present in the data presented. These include:
- Data is reported at the level of individual NHS Trusts. NHS Trusts can be made up of multiple sites. If you can't find your hospital, try searching for it by its NHS Trust name.
- NHS Trusts sometimes undergo organisational change, such as mergers or acquisitions. The hospital you are looking for could be included in the dataset under a different name to what you might expect. Data for NHS Trusts which are now part of another NHS Trust is aggregated into the successor organisation. You can find all NHS Trusts historically included in the SCMD, with an indication of their current NHS Trust on the Submission History page.
Why are there negative values for some products?
The SCMD contains pharmacy stock control data representing medication distribution within hospitals, such as issuing of medicines to wards or clinical areas, rather than actual patient-level prescribing. Not all of the stock that is issued within hospitals eventually ends up being used. In some hospitals, when this is the case and where their stock control system supports it, historical stock issues can be updated. This is known as Backtracking. Where supply made in a previous month is returned in a subsequent month, and the quantity returned is greater than the quantity issued in that month, the quantity reported will be negative.
You can read about this in our blog, More about hospital stock control data.
What is a VMP?
VMP is short for Virtual Medicinal Product. This is a component of the dictionary of medicines and devices (dm+d), the standard dictionary for medicines and devices used across the NHS and contains standardised codes, descriptions and metadata for individual items. VMPs describe a general class of medicines or device that may be available as an actual product. All of the data within the SCMD is reported at the level of VMPs. We commonly refer to these as products.
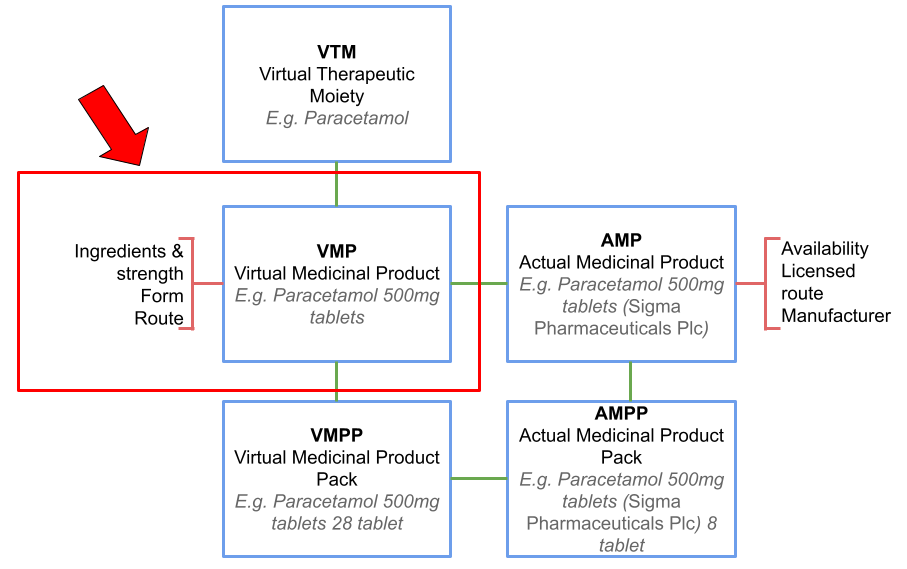
You can read more about VMPs in our blogs, Understanding the secondary care medicines dataset and Getting more from the secondary care medicines data using the dictionary of medicines and devices.
How do we identify if a VMP is unlicensed?
A VMP is identified as unlicensed when all of its associated Actual Medicinal Products (AMPs) have an availability restriction of 'Special' in the dm+d.
A VMP is marked as unlicensed only when:
- The VMP has at least one associated AMP
- All AMPs associated with the VMP have an availability restriction of 'Special'
If a VMP has multiple AMPs and only some of them are marked as 'Special', the VMP itself will not be identified as unlicensed. You can view whether a VMP is unlicensed on the Product Lookup page.
What is the Anatomical Therapeutic Chemical (ATC)/ Defined Daily Dose (DDD) system?
The ATC/DDD system is a way for classifying and measuring drug utilisation to enable comparisons of drug use between countries, regions, and other healthcare settings. It classifies medicinal products using the Anatomical Therapeutic Classification (ATC) system and provides the Defined Daily Dose (DDD) as a way to measure their usage.
It is maintained by the World Health Organisation (WHO) Collaborating Centre for Drug Statistics Methodology and is widely used internationally.
What is the Anatomical Therapeutic Chemical (ATC) system?
The Anatomical Therapeutic Chemical (ATC) system provides a classification for the active ingredients within medicinal substances based on where in the body they act and their therapeutic (what condition or disease they treat), pharmacological (how they work in the body), and chemical properties (the structure and composition of the substance).
The system is a hierarchy with five levels of increasing specificity, which you can read more about in our blog post, Classifying and measuring medicines usage with the ATC/DDD system. Below is an example of how the ATC code for olanzapine is structured:
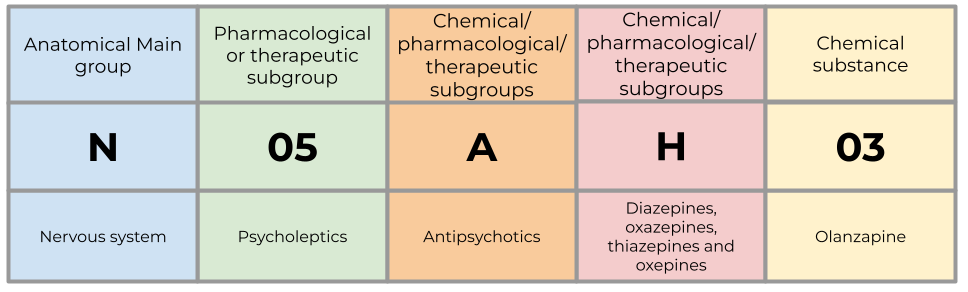
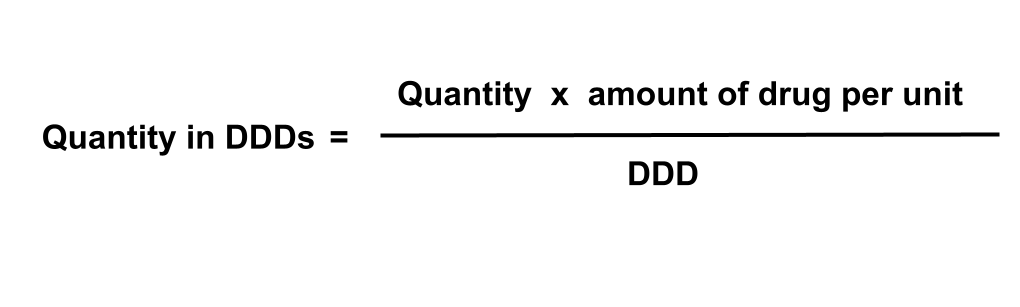
What is a Defined Daily Dose (DDD)?
The ATC system provides a way to classify drugs, but not a unit to measure them in. This is what the Defined Daily Dose (DDD) is for. DDDs are a unit of measure for medicines consumption that enables comparison of usage across groups of medicines. They are defined and maintained by the World Health Organization (WHO), who define them as:
Defined Daily Dose (DDD): The assumed average maintenance dose per day for a drug used for its main indication in adults
Source: World Health Organization
The DDD quantity for a product can be calculated as follows:
What is indicative cost?
The SCMD includes the indicative cost for each medicine or device within the dataset. The indicative cost is based on the community pharmacy reimbursement prices for generic and list prices for branded medicines. This does not reflect the actual cost paid by hospitals. This, as described in the SCMD release guidance, overestimates the total spend by a factor of two (though this overestimation is not uniform across all products):
The indicative cost in this data set will overestimate the total spend on medicines issued in hospitals. For example, the total indicative cost of medicines issued in secondary care for 2020 / 21 was £14.5 billion compared to the net actual cost of £7.59 billion (excluding central rebates).
Does the data include prescriptions dispensed in community pharmacy?
The SCMD does not include NHS prescriptions supplied by the hospital and dispensed in community pharmacy (hospital FP10 prescriptions). This data is available in a separate dataset, Hospital Prescribing Dispensed in the Community, which we do not currently use on OpenPrescribing Hospitals. Medicines dispensed in community pharmacy make up a very small proportion of hospital medicines usage; in January 2025, medicines dispensed in community pharmacy accounted for £5.6m actual cost, which is a very small proportion of the near £2.1bn indicative cost in SCMD data for the same month (likely ~£1bn actual cost).
Data quality
Why is data missing for my Trust?
The SCMD dataset relies on submission of pharmacy stock control data by individual NHS Trusts. For some NHS Trusts, submissions are not available for some months or submissions are incomplete. The data completeness for individual NHS Trusts can be found on the Submission History page.
Analyse
What does quantity mean?
Quantity is the total amount of a medicine that has been issued as reported in the SCMD. The unit of measure used to measure the quantity varies across different products. Where possible, units are mapped to a consistent unit basis (e.g. quantities reported in micrograms and milligrams are both converted to quantity in grams) to allow for comparison between different products. Quantities reported in different unit bases may not be comparable. For this reason, we calculate alternative measures of quantity from the quantity reported in the SCMD. These include SCMD quantity, unit dose quantity, ingredient quantity and DDD quantity.
Why is there no quantity for some products?
There is no quantity reported when a trust has not issued a product or the selected quantity type is not available for the product. See How is the quantity type used for an analysis chosen?.
How do I see ICB, regional, and national breakdowns?
To view ICB, regional, and national analysis breakdowns, as well as national trust-level variation as a percentiles chart, run an analysis without selecting any NHS Trusts in the analysis builder. These geographic breakdown modes are only available when analysing data across all trusts.
How do I restrict an analysis to a specific set of NHS Trusts?
To restrict an analysis to a specific set of NHS Trusts, select the NHS Trusts you want to include in the analysis builder. This will filter the analysis to show only data from your selected trusts.
What is SCMD quantity?
SCMD quantity is a normalised version of the raw quantity available in the SCMD. This quantity measure is available for all products reported in the SCMD. You can read more about how it is calculated in our blog post, Measuring quantity in the Secondary Care Medicines Data. For examples of reported SCMD quantities, search for a product on the Product Lookup page.
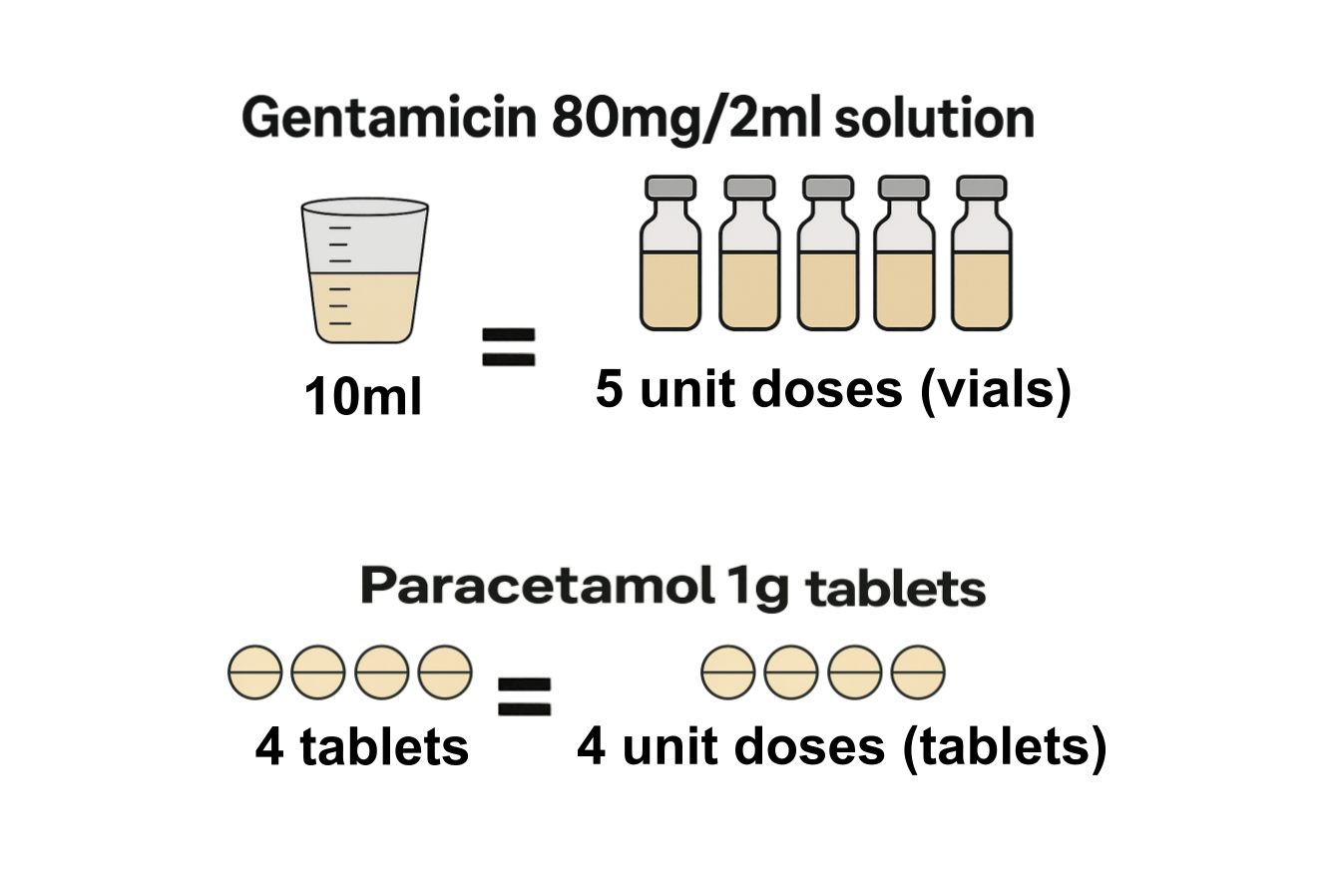
What is unit dose quantity?
A unit dose is a single measured quantity of a medicine, packaged for administration to a patient at one time. Unit dose quantity is the equivalent of the SCMD quantity in terms of the number of unit doses issued. This measure of quantity is not available for all products. You can read more about how it is calculated and the products it is available for in our blog post, Calculating unit dose quantity in the Secondary Care Medicines Data. Some example unit dose calculations are shown below. To see if, and how the unit dose quantity is calculated for any product included in the SCMD, search for a product on the Product Lookup page.
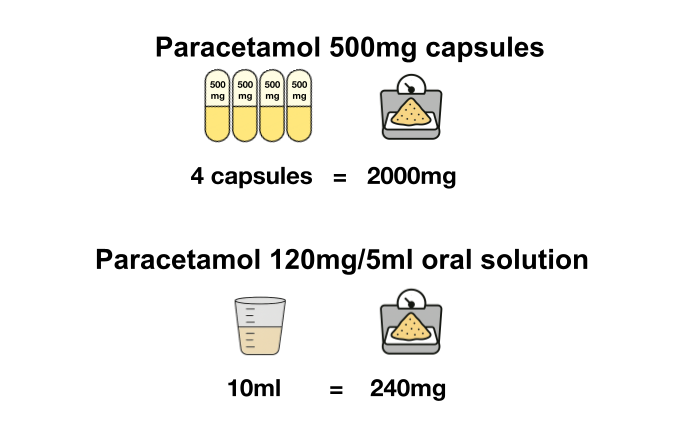
What is ingredient quantity?
Using the dm+d the individual ingredients within a product can be identified. Ingredient quantity is the quantity of each ingredient within a product that is equivalent to the SCMD quantity. Not all products have specified ingredients, and for those that do, it is not always possible to determine the ingredient quantity. You can read more about how ingredient quantity is calculated and the products it is available for in our blog post, Calculating ingredient quantity in the Secondary Care Medicines Data. Some example ingredient calculations are shown below. To see if, and how the ingredient quantity is calculated for any product included in the SCMD, search for a product on the Product Lookup page.
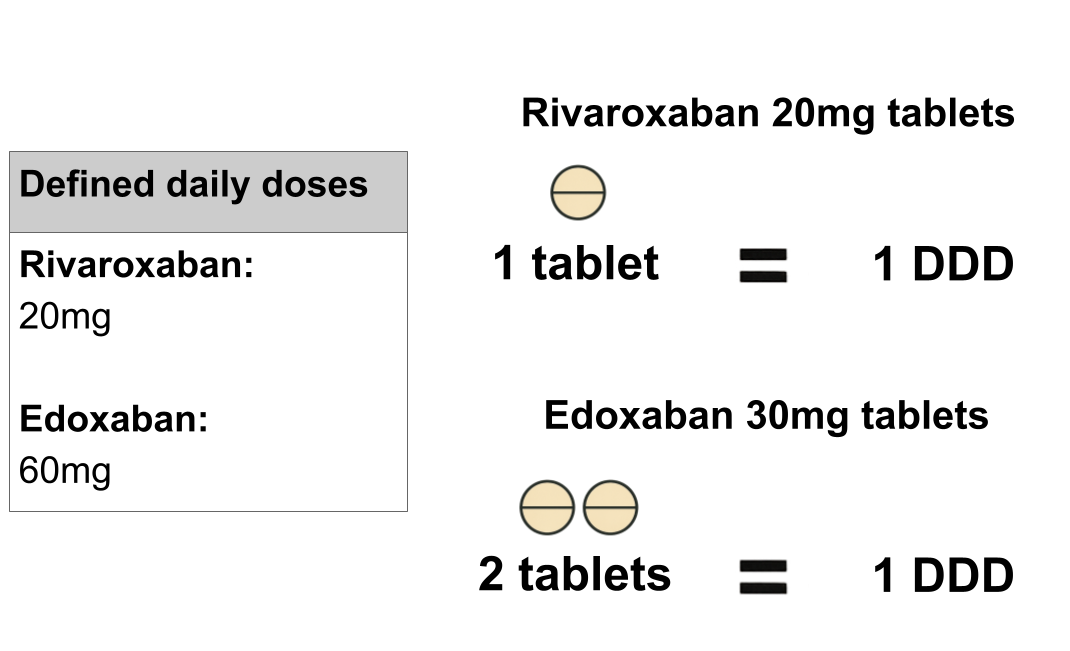
What is DDD quantity?
Defined Daily Dose (DDD) is a unit of measure for medicines consumption that enables comparison of usage across groups of medicines. DDD quantity is the equivalent of the SCMD quantity in terms of the number of DDDs issued. This measure of quantity is not available for all products. You can read more about how DDD quantity is calculated and the products it is available for in our blog post, Calculating DDD quantity in the Secondary Care Medicines Data. Some example DDD calculations are shown below. To see if, and how the DDD quantity is calculated for any product included in the SCMD, search for a product on the Product Lookup page.
How is the quantity type used for an analysis chosen?
When you select products for analysis, the most appropriate quantity type to use is automatically selected based on the information available for the products. The selection is designed to improve the likelihood that the quantities being compared are clinically meaningful and that quantity data is available for the greatest number of the selected products.
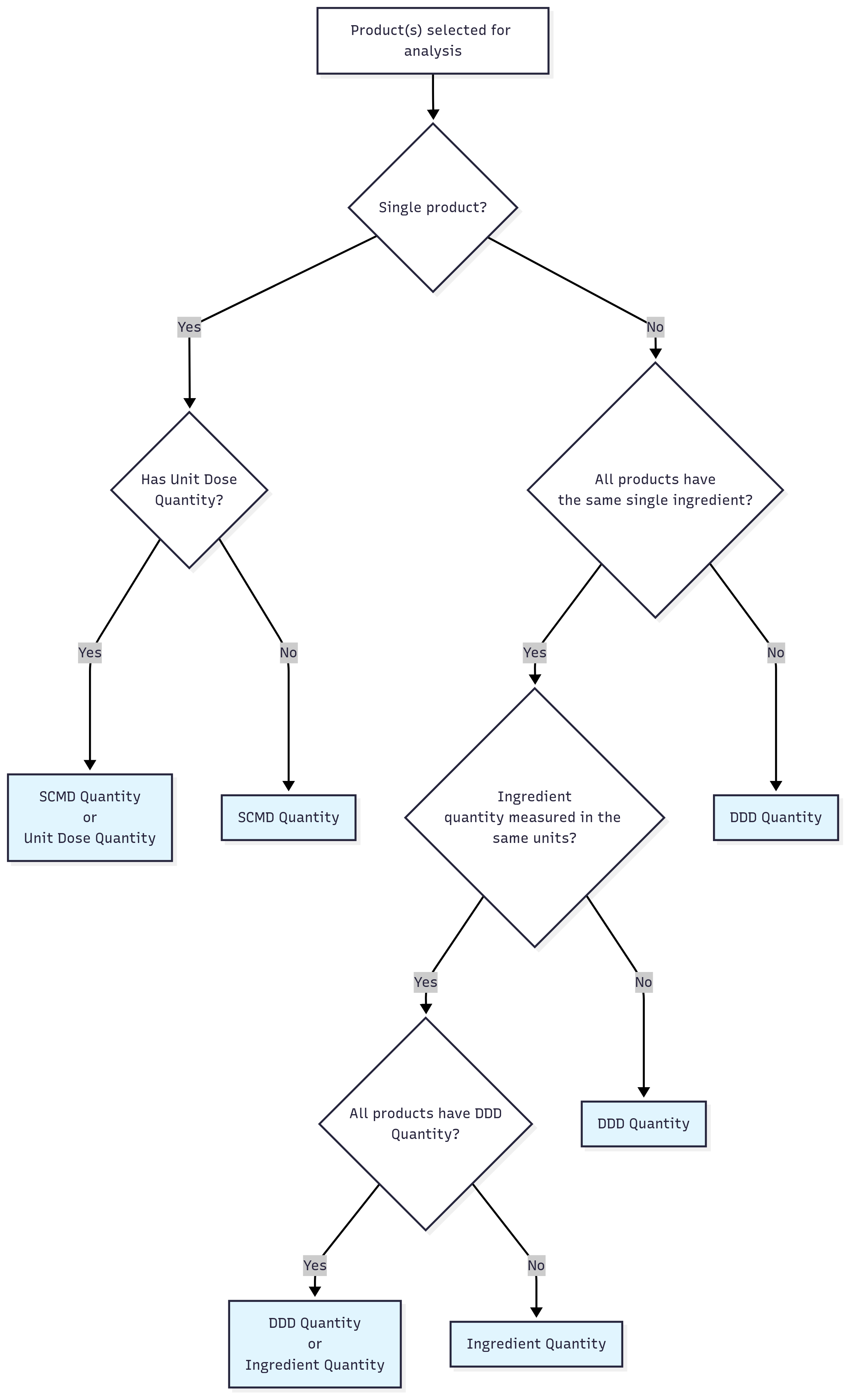
Where there is more than one recommended quantity type for a selection of products, we select a default. We do this because our user testing found that most users wanted to see a quick analysis of the data, and they were not interested in the detail of the quantity type selection. There may be some selections of products where the chosen quantity type is not what you want for your analysis. You can change the quantity type in the advanced options of the analysis builder, but pay attention to any additional warning messages indicating the appropriateness of the comparison. To see what quantity types are available for your selected products, use the Product Lookup tool.
Measures
How are measures calculated?
Measures can simply be a count of the amount of a drug being issued but more commonly a ratio is used. To calculate a ratio, we specify a list of products to include in the denominator and the subset of these products which make up the numerator. We then sum the quantity issued across the numerator and denominator separately, divide the numerator by the denominator and multiply by 100 to get a percentage.
You can read more about measures in our blog, Introducing OpenPrescribing Hospitals measures.
What are percentile charts?
Percentile charts show the extent of variation in medication use at the level of individual trusts. You can read more about why we use them in our blogs, communicating variation in prescribing and highlighting variation in hospitals medicines usage.
What does a value of zero mean in a measure?
When viewing measures, you may see trusts with a value of zero for certain months. This can happen for two main reasons:
1. No usage: The trust did not issue the relevant products during that month.
2. No data submission: The trust did not submit data for the relevant products to the SCMD for that month.
We cannot distinguish between true zeroes and zeroes resulting from missing data submissions. You can see the submission history for an trust on the Submission History page to get an idea of the completeness of the data for a specific trust.
Why do some trusts have out of range values?
Within the SCMD, it is possible for issued quantity to be negative (see Why are there negative values for some products?). When calculting measures with both a numerator and denominator, values are expected to be between 0% and 100%. However, negative values can result in the numerator being greater than the denominator (so the measure value can be greater than 100%) or the denominator being negative (so the measure value can be less than 0%).
What do annotations on measure charts show?
Measure annotations indicate events that may have influenced issuing patterns of products included a given measure. They help to provide context for changes in the data over time.
For example, annotations might mark: - Patent expiries that could affect product choice - Changes in clinical guidance - Introduction of new treatments - Policy changes affecting issuing
Can I hide annotations on measure charts?
To hide annotations on a measure chart:
- Click the chart menu in the top-right corner of the chart
- Select "Hide annotations" from the dropdown menu
To show annotations again, repeat the same steps and select "Show annotations".
Downloading Charts
How do I download a chart?
You can download charts from both the Measures pages and the Analyse tool. To download a chart:
- Click the menu icon (☰) in the top-right corner of the chart
- Select "Download PNG image" from the dropdown menu
Can I download the chart data?
You can download the underlying data for a chart as a CSV file:
- Click the menu icon (☰) in the top-right corner of the chart
- Select "Download CSV" from the dropdown menu
You can also download the raw data from which all charts are generated by clicking the menu icon (☰) in the top-right corner of any chart and selecting "Download Raw Data". This downloads a ZIP file containing CSV files with detailed analysis data.
What's included in the raw data download?
The ZIP file contains the following files:
1. Main Analysis Data CSV
This file contains the trust-level issuing quantity for each product included in an analysis.
Columns:
- Date: The month the quantity issued is aggregated over. e.g. rows where the Date is
2020-01-01represent the quantity issued across January 2020. - VMP Code: The SNOMED CT code for the Virtual Medicinal Product (VMP)
- VMP Name: The name of the Virtual Medicinal Product
- VTM Name: The name of the Virtual Therapeutic Moiety (VTM) that the VMP belongs to, if applicable
- Trust Code: The ODS (Organisation Data Service) code for the NHS Trust
- Trust Name: The name for the NHS Trust
- Region: The NHS region the trust belongs to
- ICB: The Integrated Care Board (ICB) the trust belongs to
- Value: The quantity of the VMP issued in the given month
- Unit: The unit of measure for the Value.
- Ingredients: List of active ingredient names in the product
2. Percentiles Data CSV (percentiles_data.csv)
This file is included when percentiles are available (only for analyses run across all trusts, with no specific trusts selected).
Columns:
- Date: The month for which percentiles are calculated. e.g. rows where the Date is
2020-01-01represent a percentile value, calcualted using the data for all included NHS Trusts in January 2020. - *th_percentile: Percentile values for the selected quantity across all.
Data Attribution
You are welcome to use data or graphs from this site in your academic output with attribution. Please cite:
Fisher L, Wood C, Brown A, Croker R, Black S, Curtis HJ, et al. OpenPrescribing Hospitals: An open access analytics platform for hospital medicines use in England. medRxiv; 2025. https://doi.org/10.1101/2025.11.13.25340060.
If you use data or images from this site online or in a report, please link back to us. Your readers will then be able to see live updates to the data you are interested in, and explore other queries for themselves.
Email Alerts
How do I sign up for email alerts?
You can sign up for email alerts by visiting our email alerts page.
What will I receive in the email alerts?
Our email alerts will keep you informed about:
- Data updates: When new monthly data is available from NHS Trusts
- Platform developments: New features, tools, and improvements to the platform
- Important announcements: Significant changes or updates to the service
Can I unsubscribe from email alerts?
Yes, you can unsubscribe at any time using the unsubscribe link included in every email alert we send.
Glossary
Administration form
The form in which a medicine is actually given to or taken by the patient. This may differ from the dispensing form - for example, a soluble tablet (dispensing form) becomes a solution (administration form) when dissolved in water before being taken by the patient.
dm+d (dictionary of medicines and devices)
The NHS standard dictionary for medicines and medical devices. It consists of 5 main classes of information and can be used to identify additional product information for products within the SCMD like grouping, form, chemical content, and administration route. Read more about it here.
Dispensing form
The physical form in which a medicine is supplied or dispensed. This may differ from the administration form - for example, effervescent tablets are dispensed as tablets but administered as a solution after being dissolved in water.
Dose
A specified amount of a medication to be taken at one time. E.g. 2 tablets.
Dose Form
A SNOMED CT code that represents the dispensing form of a medicine. This indicates how the medicine is packaged or dispensed, rather than how it's administered to the patient.
Dose regimen
A schedule of doses of a medicine which includes the dose, frequency and duration. E.g. 2 x 100mg tablets, twice daily, for 14 days.
Drug route
A SNOMED CT code that indicates a possible way a medicine can be administered to a patient. A VMP can have multiple possible routes of administration, such as both intramuscular and intravenous routes.
Ontology Form & Route
A dm+d-specific code that combines both the form and route information for a medicine. For example, solution.oral would be used for a soluble tablet that is taken orally.
SCMD quantity
A lightly processed version of the raw quantity reported in the SCMD. Read more about how it is generated here.
Secondary Care Medicines Data (SCMD)
A dataset containing information on the quantity of medicines and devices issued by individual NHS Trusts in England. Read more about it here.
SNOMED CT
SNOMED CT (Systematized Nomenclature of Medicine - Clinical Terms) is a structured vocabulary for recording information in patient records, mandated for use by all NHS healthcare providers in England for capturing clinical information.
Strength
Represents the amount of active ingredient in a medicinal product. Can be expressed as a single value (numerator only) or as a ratio (numerator/denominator) for solutions or concentrations. Each strength value has associated units of measure. For example, 500mg for a tablet, or 50mg/1ml for a solution.
Unit dose
Refers to a single measured quantity of medicine that is packaged and ready for patient administration. E.g, a tablet. Read more about it here.
Unit Dose Form
One of 3 possible forms for the unit dose: Discrete (for countable units like tablets), Continuous (for measurable quantities like liquids), or Not applicable (for devices like catheters where the concept of a dose isn't relevant).
Unit dose form size (UDFS)
A numeric value indicating the amount of a medicine in a unit dose.
Unit dose form unit of measure
The unit of measure for the unit dose form size.
Unit dose unit of measure
The unit of measure for the unit dose. Examples include, vial, tablet and pre-filled syringe.
VMP (Virtual Medicinal Product)
A class within the dm+d representing specific medicinal products with defined forms and strengths. VMPs contain various attributes including dose form and unit dose measurements, and link to other dm+d classes for additional information. The SCMD is reported at this level of the dm+d.
VPI (Virtual Product Ingredient)
A class within the dm+d containing detailed information about the ingredients present in a VMP. It includes strength information through a numerator (single value) and potentially a denominator (for solutions/concentrations), along with their respective units of measure.
VTM (Virtual Therapeutic Moiety)
The highest level of the dm+d hierarchy, representing an abstract concept of medical substances or devices without specifying strength or form. A single VTM can be associated with many VMPs and can represent single substances or combinations of substances.